To find the net ionic equation we must first write the balanced equation for the reaction. We must bear in mind that the reagents Ca(NO3)2 and Na2S are in the aqueous state and as product we will have CaS in the solid state, since it is not soluble in water and NaNO3 in the aqueous state.
The balanced equation of the reaction will be:
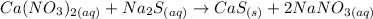
Ca(NO3)2(aq) + → Ca(aq) + 2Na(s)NO3Now, c(aq)ompounds in the aqueous state can be written in their ionic form, so the reaction will transform into:Na2S +
![Ca_((aq))^(2+)+2(NO_3)_((aq))^-+2Na_((aq))^++S_((aq))^(2-)\operatorname{\rightarrow}CaS_((s))+2Na_((aq))^++2NO^-_(3(aq))]()
So, the answer will be option A