Specific heat capacity (c) of a material is related to the Energy Absorbed (Q), mass of the material (m) and the change in temperature (T) by the following equation:
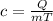
Substituting the values of Q, m and T in the above equation, we get:
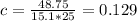
So the specific heat capacity of the metal with given conditions will be 0.129 J/g.K