Answer:
The change in entropy is
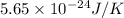 .
.
Step-by-step explanation:
The entropy can be determined from Boltzmann equation of entropy:
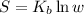
S = Entropy of the system

w = Number of microstates
1) Number of adoption sites = 484
w = 484
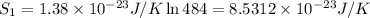
2) Number of adoption sites = 729
w =729
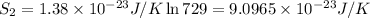
Change in entropy =
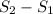
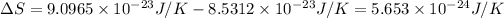
The change in entropy is
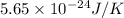 .
.