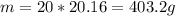Since water is already at 100
°C all the energy is used to evaporate it.
Now we can calculate how many mols of water are evaporated with 820kJ.
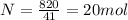
We calculated that we got 20 mols of water evaporated. Now, all we have to do is find how many grams is a mol of water. Molar mass of water is 20.16 g/mol.
The final answer is: