Answer: Option (b) is the correct answer.
Step-by-step explanation:
Molecules of a real gas are smaller is size and occupy volume or space. Real gases do not behave like ideal gases.
Mathematically, ratio of
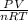 is either greater than or smaller than 1.
is either greater than or smaller than 1.
Hence, when there is increase in pressure then there will be increase in ratio. On the other hand, if there is decrease in ratio then there will be decrease in ratio.
Thus, we can conclude that for real gases, a change in pressure affect the ratio of PV to nRT because the ratio increases as pressure increases.