First, let's start off by finding the mass of this whole hydrate.
(Note: the unit of measurement for mass will be amu)
Let's find the molecular mass of each element.
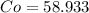
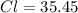
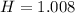
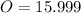
Now, let's find the mass of each compound.
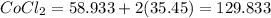
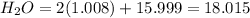
We have 6 molecules of H2O, so multiply 18.015 by 6 then add that with the weight of CoCl2.
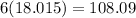
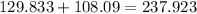
Now divide 108.09 (mass of all the H2O in the hydrate) by 237.923 (total mass of hydrate).
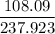
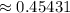
Turn that into a percentage and you get 45.431%.
Hope this helps! :)