Answer: The moles of chloride ions in sodium chloride is
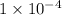 moles, in magnesium chloride is
moles, in magnesium chloride is
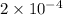 moles and in aluminium chloride is
moles and in aluminium chloride is
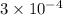 moles
moles
Step-by-step explanation:
We are given:
Moles of sodium chloride in 1 L of solution = 0.10 moles
Moles of magnesium chloride in 1 L of solution = 0.10 moles
Moles of aluminium chloride in 1 L of solution = 0.10 moles
We need to calculate the moles of chloride ion in each solution in 1 mL of solution. The conversion factor used is:
1 L = 1000 mL
1 mole of NaCl produces 1 mole of sodium ions and 1 mole of chloride ions.
Moles of chloride ions in 1 L solution = 0.10 moles
Moles of chloride ions in 1 mL solution =
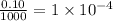 moles
moles
1 mole of
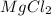 produces 1 mole of magnesium ions and 2 moles of chloride ions.
produces 1 mole of magnesium ions and 2 moles of chloride ions.
Moles of chloride ions in 1 L solution = (0.10 × 2) = 0.20 moles
Moles of chloride ions in 1 mL solution =
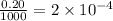 moles
moles
1 mole of
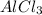 produces 1 mole of aluminium ions and 3 moles of chloride ions.
produces 1 mole of aluminium ions and 3 moles of chloride ions.
Moles of chloride ions in 1 L solution = (0.10 × 3) = 0.30 moles
Moles of chloride ions in 1 mL solution =
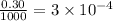 moles
moles
Hence, the moles of chloride ions in sodium chloride is
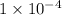 moles, in magnesium chloride is
moles, in magnesium chloride is
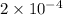 moles and in aluminium chloride is
moles and in aluminium chloride is
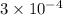 moles
moles