Answer: Option (d) is the correct answer.
Step-by-step explanation:
A strong acid is defined as the acid which completely dissociates into ions when dissolved in a solution.
For example,
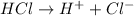
Whereas a strong base is a base which completely dissociates into its ions when dissolved in a solution.
For example,
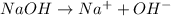
Hence, greater is the degree of ionization greater will be the strength of acid or base.
Therefore, we can conclude that with solutions of strong acids and strong bases, the word strong refer to degree of ionization.