Answer : The correct option is, carbon (C)
Explanation :
Element = Carbon
Atomic number of carbon = 6
The electronic configuration of carbon is,
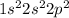
From the electronic configuration we conclude that
The number of electrons = 2 + 2 + 2 = 6 electrons
Element = Boron
Atomic number of carbon = 5
Number of electrons = 5
Element = Nitrogen
Atomic number of carbon = 7
Number of electrons = 7
Element = Potassium
Atomic number of carbon = 19
Number of electrons = 19
Therefore, the element is, carbon (C)