Step-by-step explanation:
It is known that relation between energy and wavelength is as follows.
E =
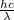
where, h = plank's constant =
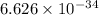
c = speed of light =
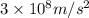
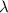 = wavelength
= wavelength
Convert nm into m as follows.
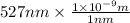
=
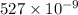 m
m
Therefore, calculate the energy as follows.
E =
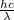
=
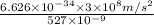
=
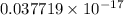 J
J
Hence, energy of the yellow light is
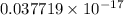 J.
J.
Now, calculate the moles of yellow light as follows.
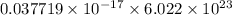
=
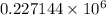 J
J
= 227144 J
or, = 227.14 kJ (as 1 kJ = 1000 J)
Thus, we can conclude that a mole of yellow photons of wavelength 527 nm has 227.14 kJ of energy.