Answer: A mole of Cu atoms has the same number of atoms as a mole of He atoms.
Step-by-step explanation:
In one mole of atoms there are
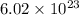 numbers of atoms. Given by Amedeo Avogadro. Also know as Avogadro number. It remain constant for every 1 mole of substance.
numbers of atoms. Given by Amedeo Avogadro. Also know as Avogadro number. It remain constant for every 1 mole of substance.
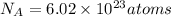
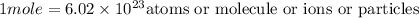
The correct statement is"A mole of Cu atoms has the same number of atoms as a mole of He atoms."