In 1 mole of
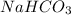 there are
there are
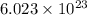 formula units of
formula units of
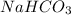 .
.
Since, 1 tsp of
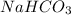 is approximately 7.20 g of
is approximately 7.20 g of
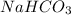 . Mass of 2.60 tsp of
. Mass of 2.60 tsp of
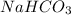 can be calculated as follows:
can be calculated as follows:
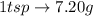
Thus,
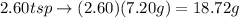
Converting mass into number of moles as follows:
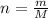
here, n is number of moles, m is mass and M is molar mass.
Molar mass of
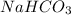 is 84 g/mol thus, number of moles will be:
is 84 g/mol thus, number of moles will be:
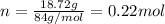
Since,
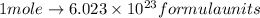
thus,
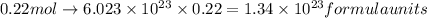
Thus,
 formula units of baking soda are in 2.60 tsp.
formula units of baking soda are in 2.60 tsp.