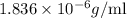Density of the liquid in units of g/ml is
Step-by-step explanation:
Density of liquid is the mass of the liquid ids divided by its volume.
Density =
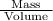
Given Mass = 4.25×108 mg
= 459mg
= 0.459g
Volume =
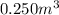
Therefore, density =
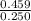
=
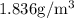
converting to
 to ml (
to ml (
 = 1000000ml)
= 1000000ml)
=