Option A: 3
The atomic number of gallium atom is 31. It belongs to 13th group and 4th period of the periodic table.
The electronic configuration is as follows:
![[Ar]3d^(10)4s^(2)4p^(1)](https://img.qammunity.org/2019/formulas/chemistry/high-school/j869195d078mrnpxjogl09vag7jkcuhm74.png)
Here, Ar is the nearest noble gas.
The outermost electronic shells are
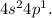
From the electronic configuration, valence electrons of gallium are 3.