Answer: 0.5 moles
Explanation: The given balanced equation is:
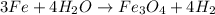
From the above equation, 4 moles of water reacts with 3 moles of Fe. So, mole ratio of water to Iron is 4:3.
If we take 2.0 moles of water and 2.0 moles of Fe then water will be the limiting reactant.
The given information says, 1.5 moles of Fe are used. We can also calculate how many moles of Fe are used as:
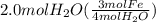 = 1.5 mol Fe
= 1.5 mol Fe
So, 2.0 moles of Fe were taken and 1.5 moles of it were used then left over moles of Fe = 2.0 moles - 1.5 moles = 0.5 moles
Hence, 0.5 moles of Fe will be left over underacted.