Answer:
14.5 moles of methane had produced 14.5 moles of carbon dioxide.
Step-by-step explanation:
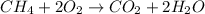
Moles of oxygen gas = 34.6 moles
According to reaction 1 mole of methane reacts with 2 moles of oxygen.
The 34.6 moles of oxygen gas will react with :
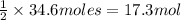 of methane
of methane
According to reaction 1 mole of methane gives 1 moles of carbon dioxide .
The 14.5 moles of carbon dioxide gas will be obtained from:
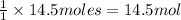 of methane
of methane
As we can see that oxygen is present in an excess , which means that formation of carbon dioxide would have depend upon moles of methane.
So, the moles of methane used to form 14.5 moles of carbon dioxide will be:
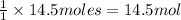 of methane
of methane