Answer: The correct answer is 2.
Step-by-step explanation:
The given chemical formula of the compound is
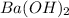
It is formed by the combination of barium atoms, oxygen atoms and hydrogen atoms.
In 1 mole of barium hydroxide, 1 mole of barium atoms, 2 moles of oxygen atoms and 2 moles of hydrogen atoms are present.
Hence, the number of oxygen atoms in 1 unit of the given compound is 2.