Answer: We require 0.16 L of HCl stock solution to make a 2 L of 1 molar HCl solution.
Step-by-step explanation:
To form a solution of 1M HCl solution from a stock solution of hydrochloric acid, we use the formula:
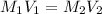
where,
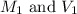 are the molarity and volume of the stock solution.
are the molarity and volume of the stock solution.
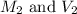 are the molarity and volume of the given HCl solution.
are the molarity and volume of the given HCl solution.
We are given:
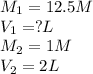
Putting values in above equation, we get:
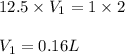
Hence, we require 0.16 L of HCl stock solution to make a 2 L of 1 molar HCl solution.