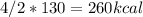So the equation is,
2Cl2(g) + 7O2(g) + 130kcal -> 2Cl2O7(g)
from this , ΔH°rxn=
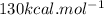
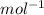 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product
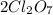 we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
Therefore