Answer:
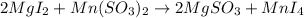
Step-by-step explanation:
Double displacement reaction: it is a chemical reaction in which the reactants exchanges their ions to form new compounds as a products.
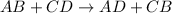
All the reaction are example of double displacement reaction beside reaction where magnesium iodide is reacting with manganese(II) sulfate to give magnesium sulfate and manganese(IV) iodide .
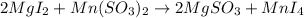
In this reaction , charge on manganese have changed from 2+ to 4+. Manganese in getting oxidized. Example of an oxidation reaction. Hence, this reaction is not an example of double displacement reaction.