Answer:
The correct answer option is 1.6 atm.
Step-by-step explanation:
We know that there is a sample gas which has a volume of 2.4 L with a pressure 1.2 atm and we are to find the pressure of the same gas sample if its volume is reduced to 1.8 L at a constant temperature.
We will apply the Boyle's law here which states that the "pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature".
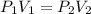
Substituting the values in the formula to get:
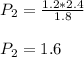
Therefore, the pressure of the same gas sample will be 1.6 atm if the volume is reduced to 1.8 L at a constant temperature.