Answer: This is known as addition reaction.
Step-by-step explanation:
Unsaturated hydrocarbon is defined as the hydrocarbons which have double or triple covalent bond present between carbon and carbon atoms.
Addition reaction is defined as the reaction in which more atoms are added to the given compound. No atom is lost during this reaction.
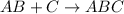
This reaction is more favored by unsaturated hydrocarbons because they are more reactive due to the presence of
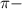 bonds. They are weaker than
bonds. They are weaker than
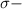 bonds and thus can be easily broken.
bonds and thus can be easily broken.
For Example: Hydrogenation of ethene molecule
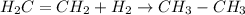
Hence, the reaction is known as addition reaction.