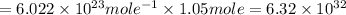Answer: Number of molecules of hydrogen gas
Step-by-step explanation:
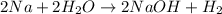
Number of moles of sodium =
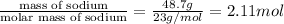
According to reaction , 2 moles of sodium produces 1 mole of hydrogen gas , then 2.11 mol of sodium will=
 of hydrogen gas that is 1.05 moles of hydrogen gas.
of hydrogen gas that is 1.05 moles of hydrogen gas.
Number of molecules =
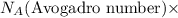 moles of substance
moles of substance
Moles of hydrogen gas formed = 1.05 moles
Number of molecules of hydrogen gas =
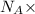 moles of hydrogen gas
moles of hydrogen gas
Number of molecules of hydrogen gas