Answer
Correct answer is :It has two elements
Explanation
A binary compound is a compound that is made up of two of the elements in the periodic table. Some examples of binary compounds are
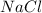 and
and
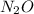 . This means that a binary compound can have more than 2 atoms as in the case of hydrogen oxide. Some binary compounds are ionic as in the case of sodium chloride.This shows that they do not necessarily have to have covalent bonds. This also rules out the possibility that they may always have double bonds as ionic compounds are bound together by ionic bonds. The only thing that will always be true of binary compounds is that they will always have 2 elements.
. This means that a binary compound can have more than 2 atoms as in the case of hydrogen oxide. Some binary compounds are ionic as in the case of sodium chloride.This shows that they do not necessarily have to have covalent bonds. This also rules out the possibility that they may always have double bonds as ionic compounds are bound together by ionic bonds. The only thing that will always be true of binary compounds is that they will always have 2 elements.