Answer: Option (a) is the correct answer.
Step-by-step explanation:
Molar mass of a substance means the sum of atomic masses of all the atoms present in a substance.
A polyethylene molecule is made up of "n" number of repeated units ethylene. Hence, it will have highest molar mass because there are more number of atoms present in a polyethylene molecule.
Whereas an ethane molecule is
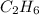 and its molar mass is
and its molar mass is
![[(12 * 2) + (1 * 6)]g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/y53k7asthven03nt5is1b0mnpoeu28p8r9.png) = 30 g/mol
= 30 g/mol
An octane molecule is
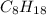 and its molar mass is
and its molar mass is
![[(12 * 8) + (1 * 18)]g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/zpbrn0ihsyxlj183eys7ek0etigxumbgmi.png) = 114 g/mol
= 114 g/mol
An octanal molecule is
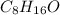 and its molar mass is
and its molar mass is
![[(12 * 8) + (1 * 16) + 16]g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/gugxz50bg1e48v3vznlbp2lailvx1jt9d0.png) = 128 g/mol
= 128 g/mol
Thus, we can conclude that polyethylene, a polymer of ethane has the highest molar mass.