Answer:- Fe changes from 0 to +3 and O changes from 0 to -2.
Explanations:- As per the rules for oxidation numbers:-
(1) Oxidation number of an atom in its elemental form is zero. Both the reactants Fe and
 are in their elemental forms and so the oxidation numbers are zero.
are in their elemental forms and so the oxidation numbers are zero.
(2) Oxidation umber of oxygen in it's compounds is -2. Let's say the oxidation number of Fe in the product is
 .
.
Also, the sum of oxidation numbers of all the atoms in a compounds is zero. So:
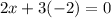
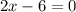
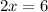
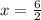
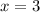
So, the oxidation number of Fe in
 is +3 and the oxidation number of O is -2.
is +3 and the oxidation number of O is -2.
Hence, the oxidation number of Fe changing from 0 to +3 and O is changing from 0 to -2.
In terms of oxidation and reduction, Iron is oxidized and oxygen is reduced.