Step-by-step explanation:
- Polar molecule are soluble in polar solvents
- Non polar molecules are soluble in non polar solvents.
Stearic acid is an organic acid composed of seventeen carbons with one carboxylic acid group in the end. Its chemical formula is
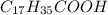 . The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.
. The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.
Where as sucrose an organic molecule with molecular formula of
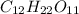 .Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.
.Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.