Answer : The pressure of the gas is, 0.964 atm
Solution : Given,
Volume of gas = 9040 ml = 9.040 L (1 L = 1000 ml)
Moles of gas = 0.447 moles
Temperature of gas =
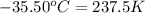
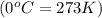
Using ideal gas equation,
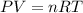
where,
P = pressure of the gas
V = volume of the gas
T = temperature of the gas
n = number of moles of gas
R = gas constant =
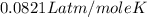
Now put all the given values in this formula, we get the pressure of the gas.
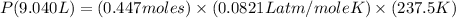
By rearranging the terms, we get
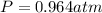
Therefore, the pressure of the gas is, 0.964 atm