Answer : The balance chemical reaction is,
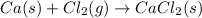
Explanation :
Balanced chemical reaction : It is defined as when the number of different atoms of elements in the reactant side is equal to that of the product side.
The balanced chemical reaction is,
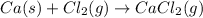
In this reaction, calcium metal react with chlorine gas to give calcium chloride.