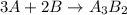Answer:-
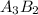
Explanations:- It is given that the charge for A is +2 and the charge for B is -3. The over all compound is neutral means the over all charge is zero. For making the over all charge zero, we need 3 positive ions and 2 negative ions. This makes a +6 charge for A and -6 charge for B and the over all charge is zero.
Also, if we think about the criss cross then charge of A becomes the subscript of B and the charge of B becomes the subscript of A.
So, the formula of the ionic compound is
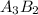 . In this compound the ratio of A to B is 3:2.
. In this compound the ratio of A to B is 3:2.
The balanced equation could be shown as: