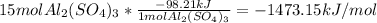The given chemical equation is:

On balancing the equation we get,

Calculating enthalpy of formation of this reaction from the standard heats of formation of the products and reactants:
Δ
![H_(reaction)^(0)</p><p>=[H_(f)^(0)(Al_(2)O_(3)(s)) + (3*H_(f)^(0)(H_(2)SO_(4)(aq))] - [H_(f)^(0)(Al_(2)SO_(4)(aq)) + (3*H_(f)^(0)(H_(2)O(l))]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/ml37j3u2ar61jf2ckgfjqh9ilp8pqkxudq.png)
=[(-1669.8kJ/mol)+ {3* (-909.27 kJ/mol)}]-[(-3442kJ/mol)+{3*(-285.8 kJ/mol)}]
=[(-4397.61kJ/mol)]-[(-4299.4kJ/mol)]
=-98.21kJ/mol
Total enthalpy change when 15 mol of
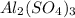 reacts will be=
reacts will be=