Answer:
When an acid is added to water, hydrogen ions Increase.
Step-by-step explanation:
When an acid is added to water it dissociates into hydrogen ions and its conjugate base:
HA ⇔
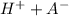
Acid ⇔ hydrogen ions + conjugate base
As the acid dissociates, the concentration of hydrogen ions increase and so do the acidity of the solution.