Answer : The value of 'R' is
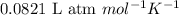
Solution : Given,
At STP conditions,
Pressure = 1 atm
Temperature = 273 K
Number of moles = 1 mole
Volume = 22.4 L
Formula used :
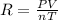
where,
R = Gas constant
P = pressure of gas
T = temperature of gas
V = volume of gas
n = number of moles of gas
Now put all the given values in this formula, we get the values of 'R'.
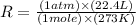
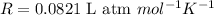
Therefore, the value of 'R' is
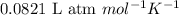 .
.