Answer:
See explanation.
Step-by-step explanation:
Hello,
21. In this case the three main indicators of a chemical change is a temperature change, a color change and/or the release of bubbles. All of them are indicators of a change in the composition of the initial reactants.
22. In this case, the correct conversion is:
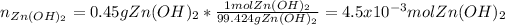
In such a way three errors are: it starts by 0.45 g not 0.65 g, zinc hydroxide's molar mass is 99.424 g/mol as its formula is Zn(OH)₂ not 82.41 g/mol and the given Avogadro's number turns needless.
23. An ion is defined as a charged species coming from the ionization of an atom or a molecule, it means, when they either lose or gain electrons depending on the case. There exist two types of ions: cations (net positive charge) and anion (net negative charge).
24.
a.
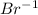 as it has seven valance electrons, it needs one electron to attain the octet.
as it has seven valance electrons, it needs one electron to attain the octet.
b.
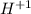 as it loses one electron when forming a cation.
as it loses one electron when forming a cation.
c.
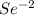 as it has six valance electrons, it needs two electrons to attain the octet.
as it has six valance electrons, it needs two electrons to attain the octet.
25.
a. Hydrazine: covalent, base.
b. Phosphorus triiodide: covalent, base.
c. Lithium hydroxide: ionic, base.
Best regards.