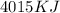Answer : The energy produced from the combustion of 5 mole
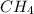 is
is
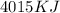
Solution : Given,
Released energy = 803 KJ
The balanced combustion reaction is,
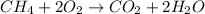
As per the question,
1 mole of
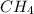 releases energy = 803 KJ
releases energy = 803 KJ
5 mole of
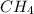 produced energy =
produced energy =
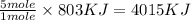
Therefore, the energy produced from the combustion of 5 mole
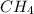 is
is