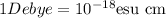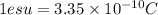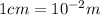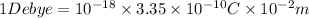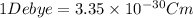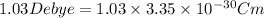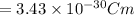Answer: Dipole moment
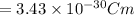
Explanation: Dipole moment is defined as product of magnitude of the charge and the distance between the charges of equal magnitude and opposite sign is called as dipole moment.
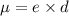
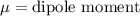
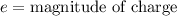
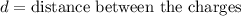
S.I unit of dipole moment is Debye (D).
Debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of
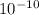 stat coulomb (generally called e.s.u. (electrostatic unit) which were separated by 1 angstrom.(1 angstrom
stat coulomb (generally called e.s.u. (electrostatic unit) which were separated by 1 angstrom.(1 angstrom
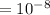 cm)
cm)