Answer:
3.67 moles
Explanation:
We need to find out the number of
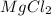 moles present in 350 grams of a compound.
moles present in 350 grams of a compound.
Molar mass of
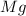 = 24.305
= 24.305
Molar mass of
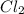 = 35.453
= 35.453
So, one mole of
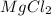 = 24.305 + (35.453 * 2) = 95.211g
= 24.305 + (35.453 * 2) = 95.211g
1 Mole in 1 molecule of
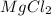 =
=
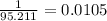
Therefore, number of moles in 350 grams of compound = 0.0105 * 350
= 3.67 moles