Answer:
K = 2 × 10⁻⁷²
Explanation:
Step 1. Determine the value of n
Zn²⁺ + 2e⁻ → Zn
2Cl⁻ → Cl₂+ 2e⁻
Zn²⁺ + 2Cl⁻ → Zn + Cl₂; E = -2.12 V
n = 2 mol electrons
===============
Step 2. Calculate K
The formula relating E and K is
E = (RT)/(nF)lnK
E = -2.12 V
R = 8.314 J·K⁻¹mol⁻¹
T = 298.15 K
n = 2
F = 96 485 C/mol
-2.12 = (8.314 × 298.15)/(2 × 96 485)lnK Do the multiplications
-2.12 = 2479/192 970 lnK Do the division
-2.12 = 0.012 85 lnK Divide both sides by 0.012 85 and transpose
ln K = -165.0 Take the antilog of both sides
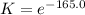
K = 2 × 10⁻⁷²