Answer:- 4.36 kPa
Solution:- At constant volume, the pressure of the gas is directly proportional to the kelvin temperature.
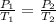
Where the subscripts 1 and 2 are representing initial and final quantities.
From given data:
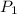 = 1.049 kPa
= 1.049 kPa
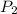 = ?
= ?
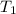 = 7.39 K
= 7.39 K
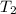 = 30.70 K
= 30.70 K
For final pressure, the equation could also be rearranged as:
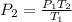
Let's plug in the values in it:
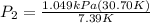
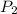 = 4.36 kPa
= 4.36 kPa
So, the new pressure of the gas is 4.36 kPa.