The complex ion has a charge of -3 which means it is an anionic complex. The nomenclature for this complex can be broken in to two parts. The part that contains the cyano
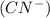 and the part that contains aluminium.
and the part that contains aluminium.
Cyano Ligand
There are 6 cyanide ions in the complex ion and so we assign the prefix hexa to explain the presence of 6 anions. This makes our ligand part be name hexacyano.
The aluminium part
Since the metal ion ends up in an anionic complex we add -ate at the end of aluminium to show that we have an anionic complex.
Henceforth we combine the two parts and start with the ligand in naming the complex.
![[Al(CN)_6]^-^3](https://img.qammunity.org/2019/formulas/chemistry/college/1bigdjwy5ml3x4lrpyfaitpphm2m4rrw8h.png) is called hexacyanoaluminate
is called hexacyanoaluminate