Answer: The structure for the boron nitride molecule will be linear.
Explanation: When boron and nitrogen combine together to form a compound, the boron element will loose 3 electrons which will be easily accepted by the nitrogen element.
Boron : Atomic number is 5
Electronic configuration:
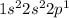
To attain stable configuration, boron will loose 3 electrons easily.
Nitrogen : Atomic number is 7
Electronic configuration:
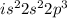
To attain stable configuration. nitrogen will gain 3 electrons easily.
Hence, to form a molecule with these two elements, there will be 3 bonds present in between them and 1 pair of lone pair will be left on the nitrogen atom.
Structure of boron nitride ( which is linear) is attached in the image below.