Answer:
6.70 g
Explanation:
A common formula for determining the amount of sample remaining in terms of its half-life is
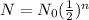
where
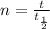
t = 458 000 yr
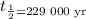 Calculate n
Calculate n
n = 458 000/229 000
n = 2.000
===============
N = 1.675 g Calculate N₀
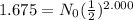
1.675 = N₀ × 0.2500 Divide by 0.2500 and transpose
N₀ = 1.675/0.2500
N₀ = 6.70 g