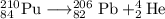Answer:
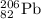
Explanation:
All isotopes with atomic numbers from 83 up are unstable.
Thus,
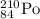 must lose at least two protons to become stable. The only common type of decay that fits is α decay, the loss of a
must lose at least two protons to become stable. The only common type of decay that fits is α decay, the loss of a
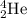 particle.
particle.
The nuclear equation is then
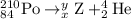
The main point to remember in balancing nuclear equations is that the sums of the superscripts and the subscripts must be the same on each side of the equation.
Then
84 = x + 2, so x = 84 - 2 = 82
210 = y + 4, so y = 210 – 4 = 206
Element 82 is lead, so the nuclear equation becomes