Answer: Option (C) is the correct answer.
Step-by-step explanation:
According to Arrhenius, bases are the species which when dissolved in water will give hydroxide ions, that is,
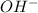 .
.
For example,
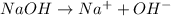
So, NaOH is a base.
On the other hand, species which tend to dissociate in water to given hydrogen ions, that is,
 ions are known as acids.
ions are known as acids.
For example,
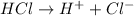
So, HCl is an acid.
Thus, we can conclude that the phrase adds hydroxide ions to the solution best describes a characteristic of an Arrhenius base.