The percent composition of Uranium in U308 = C. 81.03%
Further explanation
Given
X g of U
3.795 g of O
20.00 g of U308
Required
The percent composition of Uranium
Solution
mass of Uranium in U308 :
= mass of U308 - mass of O
= 20 g - 3.795 g
= 16.205 g
The percent composition :
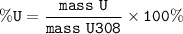
%U = (mass U/mass U308) x 100%
%U= (16.205/20) x 100%
%U = 81.025≈81.03