Answer:-
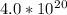 molecules.
molecules.
Solution:- The given balanced equation is:
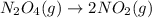
From the balanced equation, 1 molecule of the reactant gives two molecules of the product. So, the number of molecules of the products formed will be two times the molecules of the reactant.
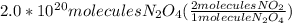
=
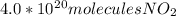
So, the last choice is the correct one.