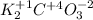Answer:
K = +1
O = -2
C = +4
Step-by-step explanation:
1. Find the oxidation number for K:
As the potassium is a metal from the group I in the periodic table, it has the number of oxidation +1
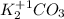
2. Find the oxidation number for O:
As the number of valence electrons of the oxygen is 6, its oxidation number is -2, that is the number of electrons left to complete the octet rule.
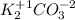
3. Find the oxidation number for C:
To find this oxidation number first multiply the oxidation numbers of the other elements by the quantity of each element in the molecular formula.
K = +1 * 2 = +2
O = -2 * 3 = -6
Therefore the oxidation number for C will be +4 because the addition of the oxidation numbers in the molecule must be equal to zero.