Answer:
![K_(eq)=([H_2]^2[O_2])/([H_2O]^2)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/ctcj3hmn0w7a9neqmo08cglymnho1co1wo.png)
Explanation:
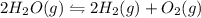
An equilibrium reaction is one in which rate of forward reaction is equal to the rate of backward reaction.
Equilibrium constant is defined as the ratio of the product of the concentration of products to the product of the concentration of reactants each raised to their stochiometric coefficient.
Thus
![K_(eq)=([H_2]^2[O_2])/([H_2O]^2)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/ctcj3hmn0w7a9neqmo08cglymnho1co1wo.png)