Answer: The correct answer is covalent bond.
Step-by-step explanation:
A covalent bond is formed when sharing of electrons takes place between the atoms that are forming a compound. These are usually formed between two non-metals.
The atoms share electrons to attain stable electronic configurations.
For Example: Formation of water molecule from the combination of hydrogen and oxygen atoms.
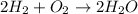
Thus, the correct answer is covalent bond.