Problem One
Formula
N(t) = No * (1/2)^[t/t_1/2]
Givens
N(t) = the current mass of the sample = 2.10 grams
No = The original mass of the sample = No [We're trying to find this].
t = time elapsed which is 2.6 billion years or 2.6 * 10^9 years.
t1/2 = the 1/2 life time which is 1.3 billion years of 1.3 *10^9
Solution
2.10 grams = No (1/2)^(2.6*10^9/1.3 * 10^9)
The 10^9s cancel and you are left with 2.6/1.3 = 2
2.10 grams = No (1/2)^2
2.10 grams = No (1/4) Multiply both sides by 4
2.10 * 4 = No (1/4)*4
8.4 grams = No
which is how many grams you originally had.
Answer B.
Problem Two

Solve for y
2 + 2 = y + 1
4 = y + 1
y = 3
Solve for z
1 + 1 = z + 0
z = 2
The 2 tells you that it is the second member on the periodic table. That's Helium. So the answer looks like this.
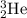
The mass of the Helium is 3 and its number is two.